How to Calculate Molar Mass of Cuso4 5h2o
Mass of water of crystallization 5 18 90. The individual molar mass of each element is given byCalcium 40078gmolBoron 1081gmolOxygen 160g.
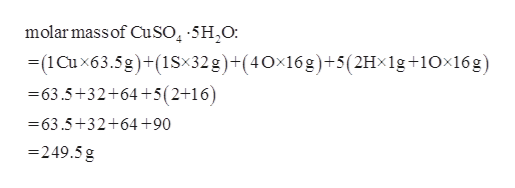
Answered Calculate The Molar Mass Of Cuso4 Bartleby
1 grams CUSO4 5H2O is equal to 00022926387885 mole.
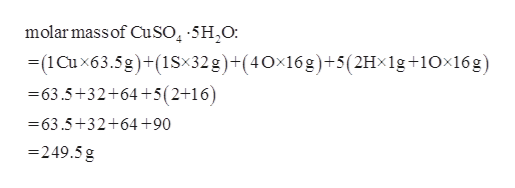
. Calculate moles Cu2 Moles Cu2 molarity Cu2 volume Cu2 Moles Cu2 010 M 0025 L. What is the color of cuso4 5h2o. 120107 23802891 32065 1599944 51007942.
CuSO45H2O CuSO4 5H2O Describe how a chemist should set up his or her stoichiometric calculation to predict the mass of CuSO4 that forms when a specified mass of CuSO45H2O is. Convert moles of CuSO45H2O to moles of CuSO4 using a mole ratio. The mass of the recovered copper is 1198 g.
Number of moles in 249 g of CuSO4. An element consists of 140 of an isotope with mass 203973 u 2410 of an isotope with mass 2059. 45 2675 Views.
Using the Periodic Table determine the sum of these masses and then plug them into the formula. Calculate the percent copper in pure copper sulfate pentahydratethis is the theoretical percent copper. It also means that copper sulfate pentahydrate contains 100 - 6392 3608 percent water by mass.
Molar mass of CuSO4 5H2O 24968 gmol. Number of moles mass molar mass 249 24972 0100 mol. 1 mole is equal to 1 moles CuSO4 or 1596086 grams.
CUSO4 5H2O molecular weight. What is the moles of CuSO4. WKT molar mass of Cuso4.
The SI base unit for amount of substance is the mole. Type in your own numbers in the form to convert the units. Hydrated copper sulfate is blue in color while.
Mass H 1 635 1 32 9 16 10 1 635 32 144 10 2495 g mol 1. Molar mass of CuSO45H2O is 2496850 gmol Convert between CuSO45H2O weight and moles. Molecular mass of CuSO4 atomic mass of copper atomic mass of Sulphur 4 x atomic mass of Oxygen 635 32 64 1595 u.
The mass of the CuSO4 x 5H2O sample is 1664 g. Note that rounding errors may occur so always check the results. Molar mass of CuSO 45H 2 O 2495 g mol 1.
Molar mass of CuSO45H2O atomic mass of Cu atom mass of S 4 atomic mass of O 5 2 atomic mass of H atomic mass of O. Using a periodic table look up the molar masses of the elements. Hydrated ionic compounds are ionic compounds with the stated number of water molecules included into the interstitial sites of the ionic lattice.
Convert moles of CuSO4 to mass of CuSO4 using molar mass of CuSO4. In hydrated compounds a specific number of water molecules becomes part of the lattice as a result you must account for the indicated molecules of water in calculating. Number of moles mass molar mass 249 24972 0100 mol.
For 1 mol CuSO4 5H2O we have 1 mol Cu2 For 00025 moles Cu2 well have 00025 moles CuSO4 5H2O. Mass S 9 At. Calculate moles CuSO4 5H2O needed.
Well there are several steps involved when calculating a molar mass. One mole of copper has a mass of 6355 g and by. Convert grams CUSO4 5H2O to moles or moles CUSO4 5H2O to grams.
Mass O 10 At. Number of moles mass molar mass 249 24972 0100 mol. Molar mass of CUSO4 5H2O 43617861 gmol.
H hydrogen Cl chlorine. The relative formula mass of CuSO45H2O is worked out from the following massesCu 6355S 3206O x 9 16 x 9 144H x 10 1 x 10 10Adding these up we get a relative formula mass of 24961. Log in or register to post comments.
Percent water mass of watermass CuSO45H2O x 100. Enter a chemical formula to calculate its molar mass and elemental composition. Calculate the molar mass of CuSO4 5H2O show your work in details.
Mass Cu 1 At. Use this page to learn how to convert between grams CUSO4 5H2O and mole. Moles Cu2 00025 moles.
Identify the different chemical elements. Also Know what is the mass percentage of water in copper sulfate. Show your work with details.
When copper II sulfate pentahydrate CuSO45H2O is heated the water is driven off leaving behind solid copper II sulfate CuSO4 according to the equation below. When a 1000 g sample of CuSO 4 5 H 2 Os was heated so that the waters of hydration were driven off the mass of the anhydrous salt remaining was found to be 06390 g. Calculate the molar mass of CuSO4 5H2O.
What is the mass of anhydrous CuSO4. Experts are tested by Chegg as specialists in their subject area. What is the percentage of water of crystallization in CuSO4 5h2o.
Molar mass of CuSO 45H 2 O 1 At. Who are the experts. How to find the molar mass of cuso4.
Add the atomic masses of Cu and S and 4 times O. So the molar mass of CuSO4 63546 3206 415999 1596. From a periodic table the masses are Cu 63546.
Write down the chemical formula. The total mass of the compound includes the 5 moles of water plus 1 mole of Cu 1 mole of S and 4 moles of Oxygen. Number of moles in 249 g of CuSO4.
Thus 1 mole of CuSO4 weighs 1596 grams. 5H2O 159609 90 250. Calculate the number of moles in 250 grams of CuSO45H2O.
How many moles of water are in CuSO4. How many moles of water will be there. Click to see full answer.

Molar Mass Molecular Weight Of Cuso4 5h2o Copper Ii Sulfate Pentahydrate Youtube
Q3 A Calculate The Molecular Mass Of Cuso4 5h2o Atomic Mass Cu 63 5u S 32u O 16u H 1u B Calculate The Number Of Molecules Present In 9 G Of Water Snapsolve

No comments for "How to Calculate Molar Mass of Cuso4 5h2o"
Post a Comment